CDC recommends Pfizer, Moderna COVID-19 shots over J&J’s
- Share via
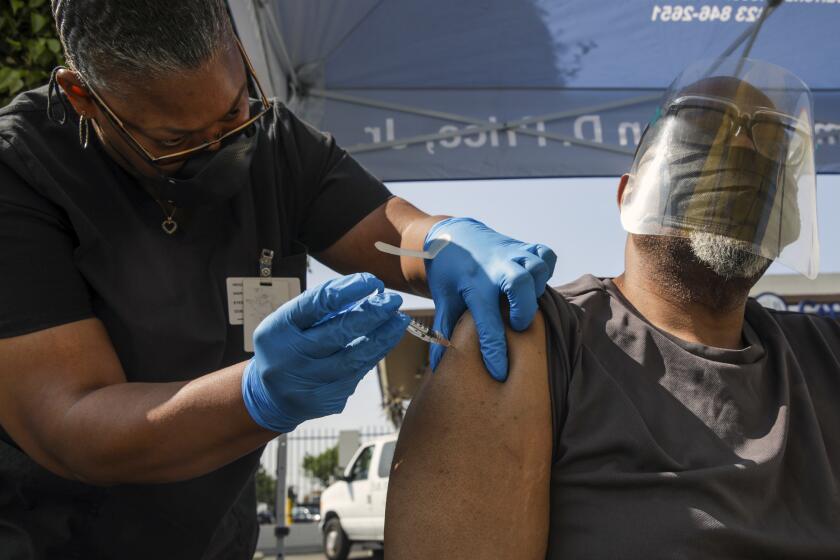
Most Americans should be given the Pfizer-BioNTech or Moderna vaccines instead of the Johnson & Johnson shot that can cause rare but serious blood clots, U.S. health officials said Thursday.
The unusual clotting problem has caused nine confirmed deaths after J&J vaccinations, while the Pfizer and Moderna vaccines don’t come with that risk and also appear more effective, advisors to the Centers for Disease Control and Prevention concluded earlier in the day.
The panel recommended the unusual move of giving preference to the Pfizer and Moderna vaccines, and the CDC’s director, Dr. Rochelle Walensky, accepted that advice.
Until now, the U.S. has treated all three COVID-19 vaccines available to Americans as an equal choice, since large studies found they all offered strong protection against COVID-19 and early supplies were limited. J&J’s vaccine initially was welcomed as a single-dose option that could be especially important for hard-to-reach groups such as homeless people who might not get the needed second dose of the Pfizer or Moderna options.
But the CDC’s advisors said Thursday that it was time to recognize a lot has changed since vaccines began rolling out a year ago. More than 200 million Americans are considered fully vaccinated, including about 16 million who got the J&J shot.
New data from unprecedented safety tracking of all those vaccinations convinced the panel that while the blood clots linked to J&J’s vaccine remain very rare, they’re still occurring and not just in younger women as originally thought.
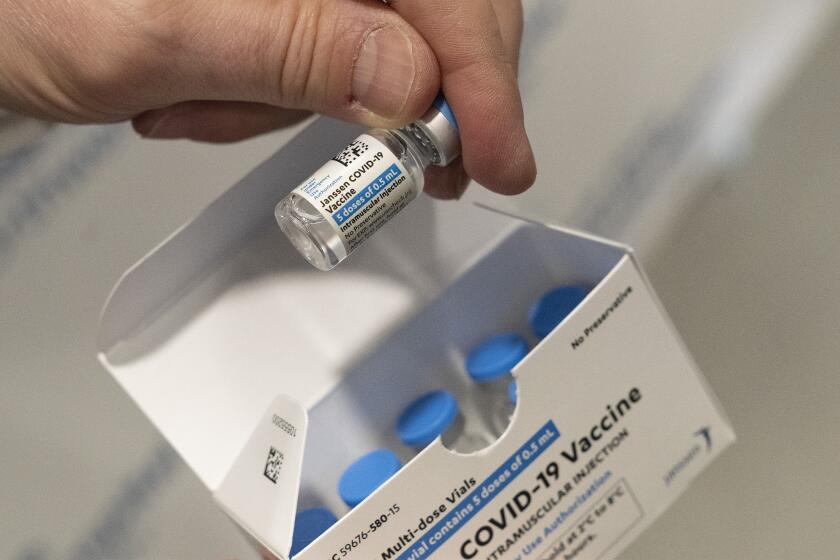
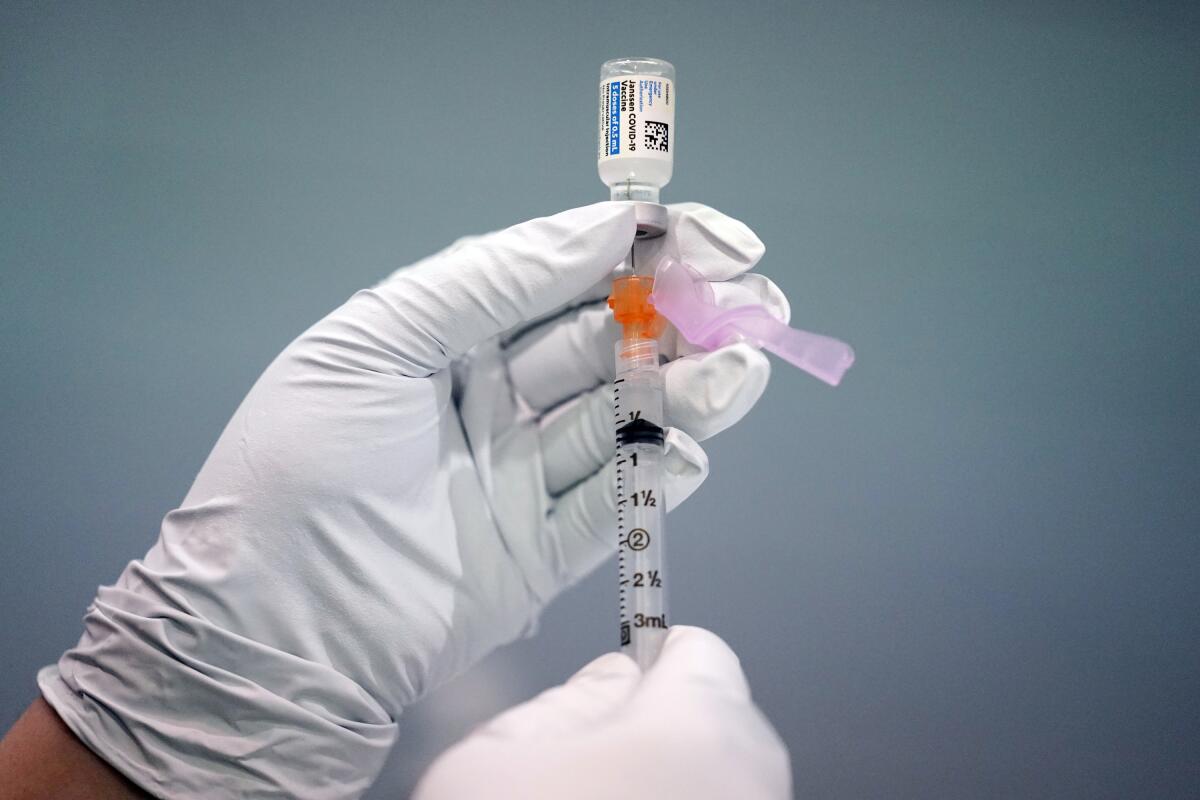
In a unanimous vote, the advisors decided the safer Pfizer and Moderna vaccines are preferred. But they said the shot made by J&J’s Janssen division still should be available if someone really wants it — or has a severe allergy to the other options.
Having side effects after getting a COVID-19 vaccine are a sign that your immune system is responding, experts say.
“I would not recommend the Janssen vaccine to my family members,” but some patients may — and should be able to — choose that option, said Dr. Beth Bell, a CDC advisor from the University of Washington.
The clotting problems first came up in the spring, with the J&J shot in the U.S. and with a similar vaccine made by AstraZeneca that is used in other countries. Eventually U.S. regulators decided the benefits of J&J’s one-and-done vaccine outweighed what was considered a very rare risk — as long as recipients were warned.
European regulators likewise continued to recommend AstraZeneca’s two-dose vaccine, but because early reports mostly involved younger women, some countries issued age restrictions.
COVID-19 causes deadly blood clots too. But the vaccine-linked kind is different, believed to form because of a rogue immune reaction to the J&J and AstraZeneca vaccines that’s a consequence of how they’re made. It forms in unusual places, such as veins that drain blood from the brain, and in patients who also develop abnormally low levels of the platelets that form clots.
Symptoms of the unusual clots, dubbed thrombosis with thrombocytopenia syndrome, include severe headaches a week or two after the J&J vaccination — not right away — as well as abdominal pain and nausea.
The CDC and FDA lifted their pause on Johnson & Johnson vaccinations, clearing the way for states to resume shots despite the tiny risk of rare blood clots.
Although clots are still very rare, the Food and Drug Administration told healthcare providers this week that more cases have occurred after J&J vaccinations since the spring. They occur mostly in women ages 30 to 49 — about once for every 100,000 doses administered, the FDA said.
Overall, the government has confirmed 54 clot cases — 37 in women and 17 in men — and nine deaths, which included two men, Dr. Isaac See of the CDC said Thursday. He said two additional deaths are suspected.
The CDC decides how vaccines should be used in the U.S., and its advisors called the continuing deaths troubling. In comparing the pros and cons of all the vaccines, the panelists agreed that side effects from the Pfizer and Moderna vaccines weren’t as serious — and that supplies now are plentiful.
Nor is J&J still considered a one-and-done vaccine, several advisors noted. The single-dose option didn’t provide quite as much protection as two doses of the Pfizer and Moderna vaccines. Plus, with extra-contagious variants spreading, booster doses now are recommended.
Several countries, including Canada, already have policies that give preference to the Pfizer and Moderna vaccines. But J&J told the committee that its vaccine still offers strong protection and is a critical option, especially in parts of the world without plentiful vaccine supplies or for people who don’t want a two-dose shot.
Although blood clots are rare, “unfortunately cases of COVID-19 are not,” said Dr. Penny Heaton of Johnson & Johnson.
The first full year of the biggest vaccination drive in American history has saved many lives but has left many behind.
The U.S. is fortunate in its vaccine availability and Thursday’s action shouldn’t discourage use of J&J’s vaccine in places around the world where it’s needed, said Dr. Matthew Daley of Kaiser Permanente Colorado, a member of the advisory committee.
The FDA also warned this week that another dose of the J&J vaccine shouldn’t be given to anyone who developed a clot after either a J&J or AstraZeneca shot.
The committee also heard some of the first data on reported side effects of Pfizer vaccinations in younger children. Early last month, the CDC recommended a two-dose series for children ages 5 to 11, and more than 7 million doses have been given so far.
Few problems have been reported: Of the 80 reported cases of serious side effects, about 10 involved a form of inflammation that has been seen in male teens and young adults.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.