AstraZeneca asks FDA to authorize COVID antibody treatment

- Share via
LONDON — AstraZeneca, the drugmaker that developed one of the first COVID-19 vaccines, has asked the U.S. Food and Drug Administration to authorize the emergency use of a first-of-a-kind antibody treatment to prevent the disease.
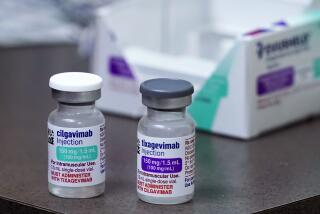
The Anglo-Swedish company said Tuesday that the treatment, known as AZD7442, would be the first long-acting antibody combination to receive an emergency authorization for COVID-19 prevention. If authorized, the drug would probably be limited to people with compromised immune systems who don’t get sufficient protection from vaccination.
“First and foremost we want to protect those vulnerable populations that haven’t been adequately protected by the vaccine,” said Menelas Pangalos, AstraZeneca’s head of research and development. “But ultimately it will be up to health authorities to work out who they choose to immunize.”
Pangalos said the company’s long-acting formulation is designed to boost immunity for up to one year, compared with existing drugs that offer a month or two of protection.
U.S. regulators said transplant recipients and others with severely weakened immune systems can get an extra dose of the Pfizer or Moderna vaccines.
The FDA has authorized three other antibody drugs, including two that can be given after a possible COVID-19 exposure to head off symptoms. AstraZeneca’s drug would instead be given as a preventive measure in people who have increased vulnerability to the virus.
The FDA has stressed that antibody drugs are not a substitute for vaccination, which is the most effective, long-lasting form of virus protection. Antibody drugs also are expensive to produce and require an IV or injection and healthcare workers to administer.
Late-stage human trials showed that AstraZeneca’s antibody drug reduced the risk of developing symptomatic COVID-19 by 77%. More than three-quarters of the participants had suppressed immune systems due to cancer, lupus and other conditions that made them more susceptible to severe disease.
Pangalos said the company’s drug will provide “an additional option to help protect against COVID-19 alongside vaccines.” The company will also seek regulatory authorization in Europe and other regions across the world.
Drugmaker Merck says its experimental COVID-19 pill reduced hospitalizations and deaths by half in people recently infected with the coronavirus.
The drugs are laboratory-made versions of virus-blocking antibodies that help fight off infections. The treatments help the patient by supplying concentrated doses of one or two antibodies.
U.S. demand for the treatments soared over the summer, particularly in states such as Florida, Louisiana and Texas, where unvaccinated patients threatened to overwhelm hospitals.
The main antibody treatments being used in the U.S. are from Regeneron and Eli Lilly & Co. The U.S. government has purchased bulk quantities of both drugs and oversees their distribution to the states.
AstraZeneca said it is in purchase talks with the U.S. and other governments around the world. If authorized, Pangalos said, the company is capable of producing dose quantities in the “low millions.”
Kirka reported from London and Perrone from Washington.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.