Costa Mesa lab retooled to test for coronavirus, now reads 1,000 tests a day and is ready for more

- Share via
March 13 was an unprecedented day for reproductive endocrinologist Dr. Bill Yee, whose practice had to close four fertility clinics in Irvine, Redondo Beach, Westminster and Beverly Hills due to the spread of the novel coronavirus.
Physicians with Reproductive Partners Medical Group treated patients finishing up therapy cycles, then closed shop for the foreseeable future — the first such interruption in the group’s nearly 35 years of practice.
“I don’t think anybody was prepared for this,” Yee said of the pandemic. “We lived through 9/11. We lived through the Great Recession. But who ever thought that a virus was going to shut down not only the United States but the world?”
Although medical clinics may continue to operate during California’s shelter-in-place restriction, Yee and his colleagues didn’t feel it was safe to potentially expose clinic staff and patients to the risk of the virus without a fast, reliable testing regimen in place.
“We felt in order for us to reopen our practice we needed testing,” Yee said. “The problem with testing was that it was very limited — even if it was available, to get the results back would take three or four days.”
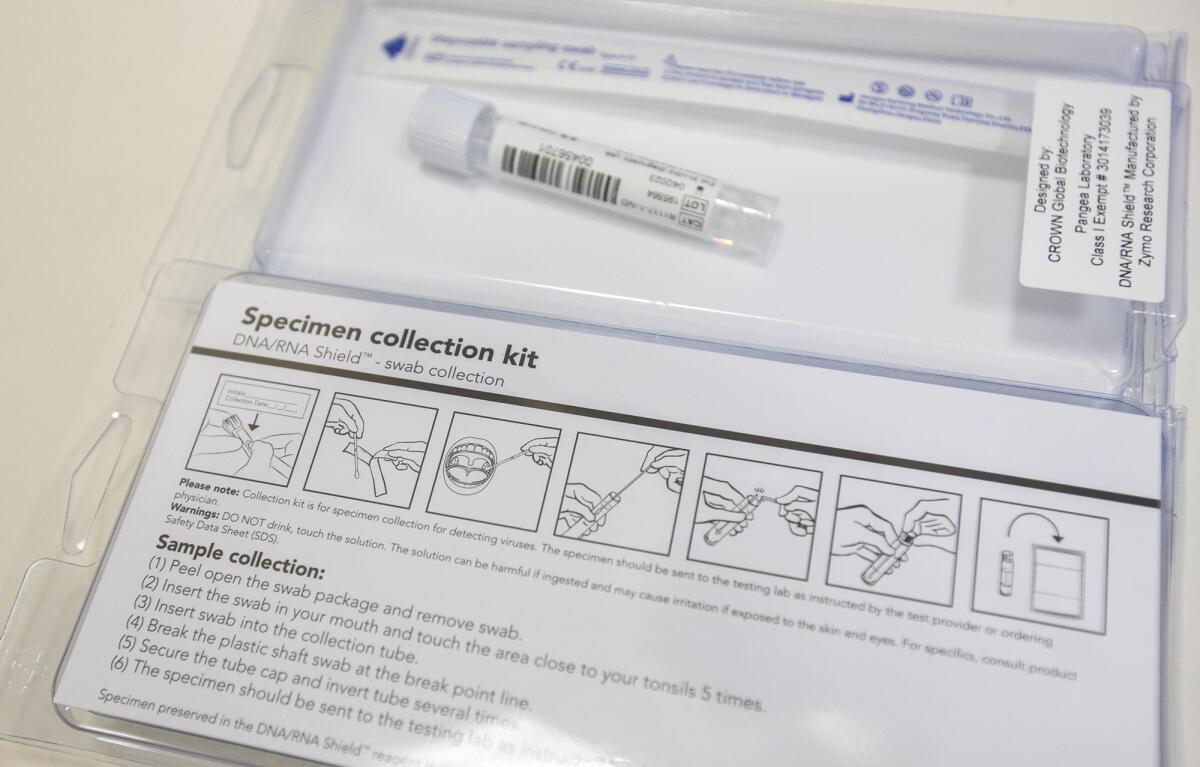
One large company the clinic worked with, for example, could only supply 10 test kits. Yee estimated the four clinics would need about 400 kits to test patients upon intake and again right before treatment.
A glimmer of hope arrived when one of Yee’s employees mentioned his son worked for a diagnostic laboratory in Costa Mesa that was certified to test for COVID-19 and could deliver results within 24 hours.
Founded in 2014, Pangea Laboratory is an accredited and certified diagnostics company focused on the early detection of emergent diseases.
Email [email protected] to sign up for the newsletter featuring the latest news involving Newport Beach, Huntington Beach, Costa Mesa, Laguna Beach, Fountain Valley and other parts of Orange County.
In January, a staff of about 15 scientists had been working with Irvine sister company Zymo Research — a manufacturer of biomedical tools and DNA/RNA extraction kits — testing for bladder cancer, when news of a coronavirus spreading throughout China began to break.
Dr. Janina Krumbeck, director of microbiome applications for Zymo, said the Costa Mesa lab was already equipped to perform molecular diagnostic testing for SARS-CoV-2 and decided to retool its operations to respond to what appeared to be a growing crisis.
“We had all the infrastructure and background to respond to a pandemic like this pretty quickly,” Krumbeck said. “The COVID test itself is a real-time PCR test. Our personnel are already highly trained to read those tests.”

Pangea has four polymerase chain reaction (PCR) machines capable of making copies of RNA from small samples, which can then be amplified for the detection of certain signals that indicate the presence of a disease or virus, like SARS-CoV-2.
Pangea received approval through the U.S. Food and Drug Administration to offer laboratory-developed tests for coronavirus in mid-April and is now offering SARS-CoV-2 detection testing using Zymo Research’s authorized EUA workflow.
The lab runs about 270 samples on each of its PCR machines each shift, amounting to more than 1,000 tests per day. Krumbeck said if needed, Pangea could access enough personnel and equipment to double its output.
“We’re just a small lab, so maybe people aren’t aware of us yet,” she said. “[But] we have the capacity, and we’re happy to help.”
Californians have been rushing to increase the number of coronavirus testing facilities as health experts and government leaders deliberate whether and how businesses might safely reopen and begin to revitalize the economy and job market.
The California Department of Public Health reported Wednesday a cumulative total of 1,104,651 tests had been reported statewide, marking an increase of 39,059 tests over the prior 24-hour reporting period — a far cry from the short-term goal of 60,000 to 80,000 daily tests set by Gov. Gavin Newsom in late April.
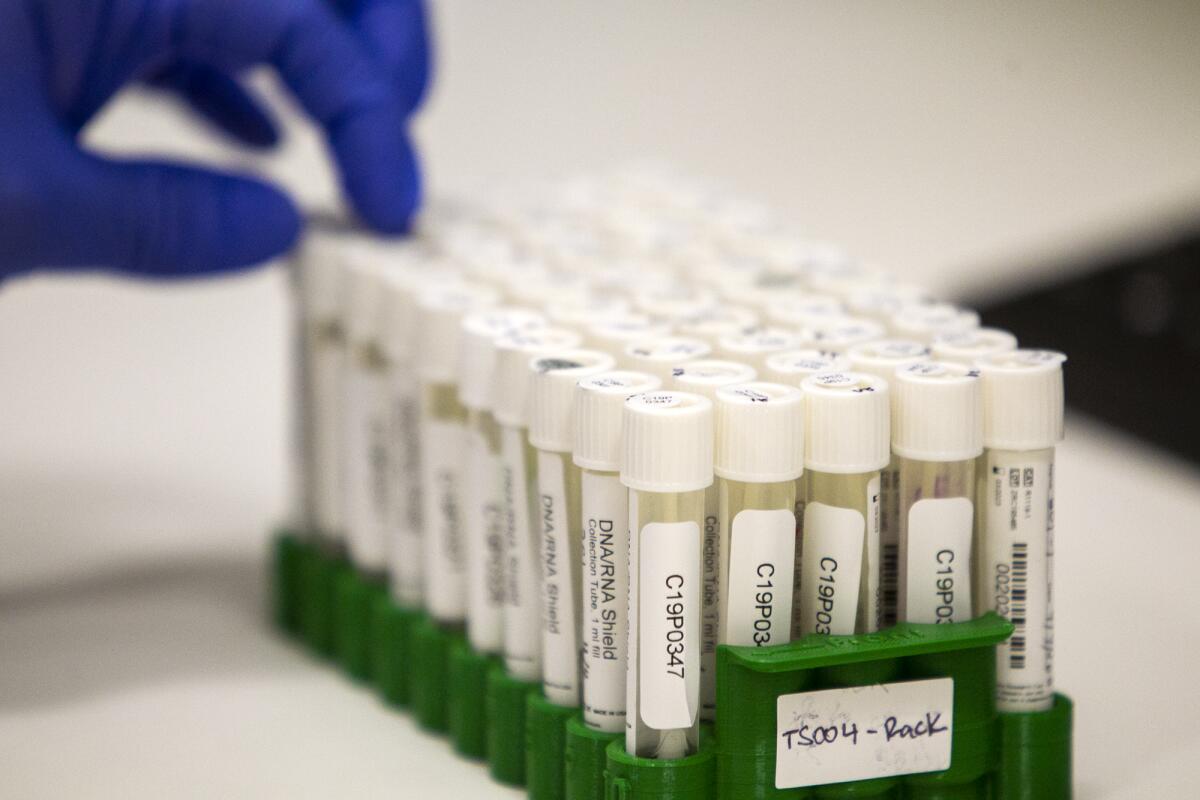
Locally, the Orange County Health Care Agency reported Thursday 61,619 cumulative tests have been performed countywide with 1,510 daily tests reported as of noon.
Krumbeck said increased testing among all residents will be a crucial part of understanding and controlling the virus.
“Testing is absolutely a next phase forward out of this pandemic,” the biologist said. “It’s so important we keep tracking everyone who is asymptomatic or symptomatic — the more we can test, the better.”
For Yee, testing has brought some relief. Three weeks ago, after Reproductive Partners Medical Group reached out to Pangea, it reopened its clinics and returned 150 staff members to work.
Now, the firm tests all patients twice and employs distancing and temperature tests for clients and employees, as recommended by the American Society for Reproductive Medicine.
If an employee isn’t feeling well or has a fever, physicians can often get a preliminary result from Pangea that evening and determine the best course of action.
“Having these tests that give us rapid results has allowed us to have a safer environment,” Yee said. “It allows us to feel more comfortable for both our patients as well as our staff, and it allowed us to reopen quickly.”
All the latest on Orange County from Orange County.
Get our free TimesOC newsletter.
You may occasionally receive promotional content from the Daily Pilot.





