FDA restricts Johnson & Johnson’s COVID-19 vaccine due to blood clot risk
- Share via
WASHINGTON — U.S. regulators on Thursday strictly limited who can receive Johnson & Johnson’s COVID-19 vaccine due to the ongoing risk of rare but serious blood clots.
The Food and Drug Administration said the shot should be given only to adults who cannot receive a different vaccine or specifically request J&J’s vaccine. U.S. authorities for months have recommended that Americans starting their COVID-19 vaccinations use the Pfizer or Moderna shots instead.
FDA officials said in a statement that they decided to restrict J&J’s vaccine after taking another look at data on the risk of life-threatening blood clots within two weeks of vaccination.
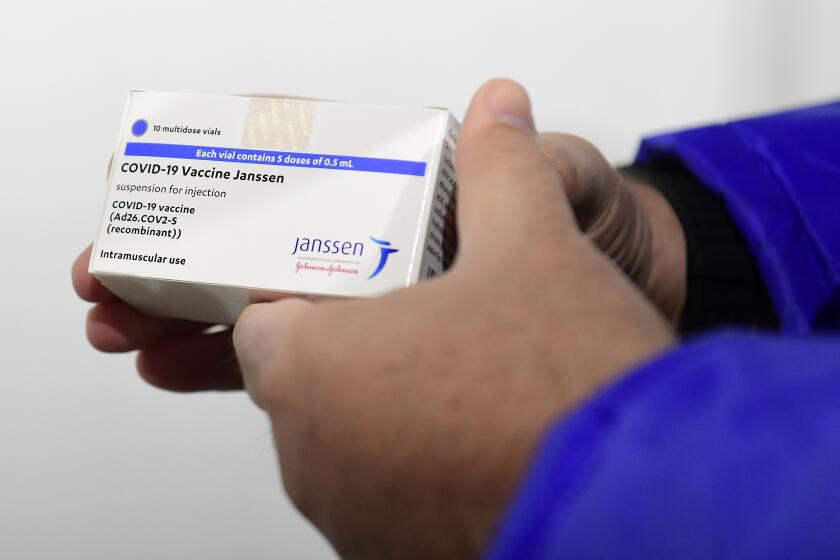
J&J’s vaccine was initially considered an important tool in fighting the pandemic because it required only one shot. But the single-dose option proved less effective than two doses of the Pfizer and Moderna vaccines.
In December, the U.S. Centers for Disease Control and Prevention recommended prioritizing Moderna and Pfizer shots over J&J’s because of its safety issues. Previously, U.S. officials had treated all three vaccines similarly because they’d each been shown to offer strong protection.
But follow-up studies have consistently shown lower effectiveness for J&J’s vaccine. And while the blood clots seen with J&J’s shot are rare, officials say they’re still occurring.
The virus is mutating rapidly and is likely to render vaccines and treatments less and less effective.
Federal scientists identified 60 cases, including nine that were fatal, as of mid-March. That amounts to one blood clot case per 3.23 million J&J shots administered, the FDA said Thursday. The vaccine will carry a starker warning about potential “long-term and debilitating health consequences” of the side effect.
Under the new FDA instructions, J&J’s vaccine could still be given to people who had a severe allergic reaction to one of the other vaccines and can’t receive an additional dose. J&J’s shot could also be an option for people who refuse to receive the mRNA vaccines from Pfizer and Moderna and therefore would otherwise remain unvaccinated, the agency said.
A J&J spokesman said in an emailed statement: “Data continue to support a favorable benefit-risk profile for the Johnson & Johnson COVID-19 vaccine in adults, when compared with no vaccine.”
Despite the restriction, the FDA’s vaccine chief, Dr. Peter Marks, said J&J’s vaccine “still has a role in the current pandemic response in the United States and across the global community.”
The FDA based its decision on “our safety surveillance systems and our commitment to ensuring that science and data guide our decisions.”
The clotting problems first came up last spring, with the J&J shot in the U.S. and with a similar vaccine made by AstraZeneca that is used in other countries. At that time, U.S. regulators decided the benefits of J&J’s one-and-done vaccine outweighed what was considered a very rare risk — as long as recipients were warned.
The Food and Drug Administration has set tentative dates in June to publicly review COVID-19 vaccines for the youngest American children.
COVID-19 causes deadly blood clots, too. But the vaccine-linked kind is different, believed to form because of a rogue immune reaction to the J&J and AstraZeneca vaccines because of how they’re made. It forms in unusual places, such as veins that drain blood from the brain, and in patients who also develop abnormally low levels of the platelets that form clots. Symptoms of the unusual clots include severe headaches a week or two after the J&J vaccination — not right away — as well as abdominal pain and nausea.
The New Brunswick, N.J.-based company announced last month that it didn’t expect a profit from the vaccine this year and was suspending sales projections.
The rollout of the company’s vaccine was hurt by a series of troubles, including manufacturing problems at a Baltimore factory that forced J&J to import millions of doses from overseas.
Additionally, regulators added warnings about the blood clots and a rare neurological reaction called Guillain-Barré syndrome.
Pfizer and Moderna have provided the vast majority of COVID-19 vaccines in the U.S. More than 200 million Americans have been fully vaccinated with the companies’ two-dose shots, while less than 17 million Americans got the J&J shot.
Why might some COVID-19 vaccines pose a small risk of blood clots while others don’t? Scientists suspect it’s related to their use of adenoviruses.