Vaccine for kids under 5 is delayed after FDA review stalls
- Share via
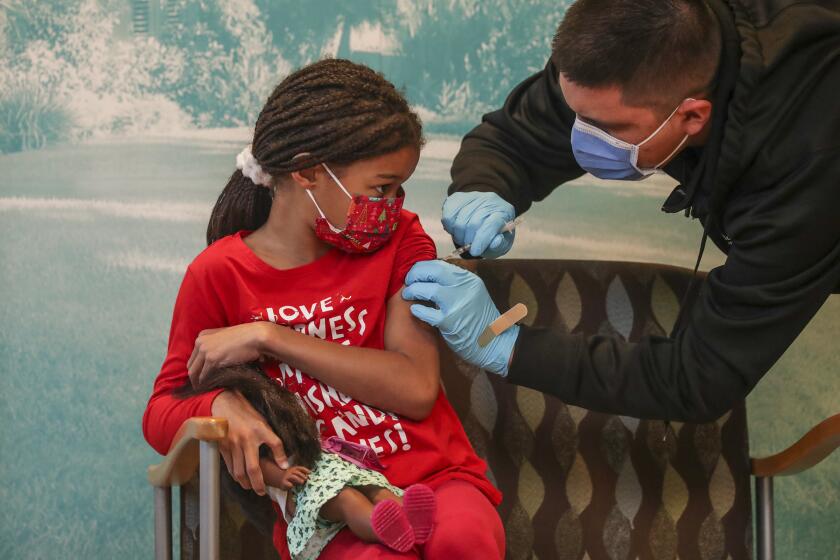
A COVID-19 vaccine for children under age 5 is unlikely to be cleared for use in the U.S. until the spring after federal regulators opted to postpone a review scheduled for next week to wait for more data.
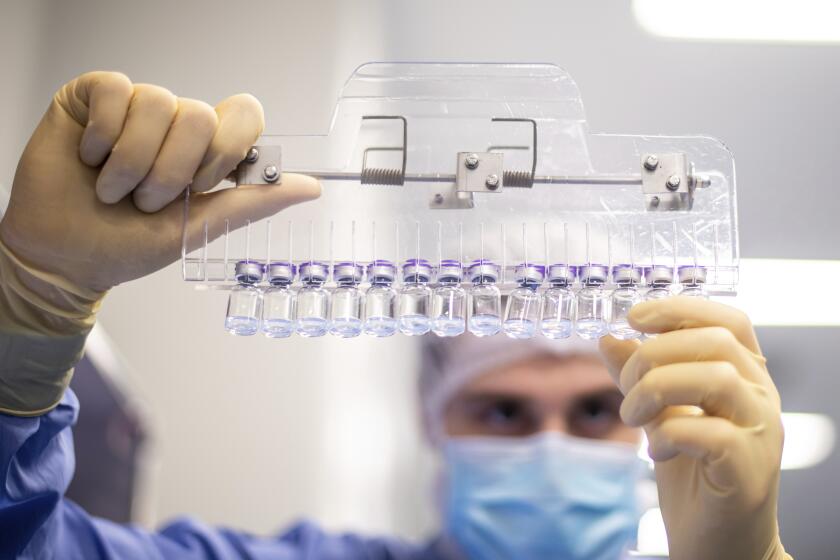
Food and Drug Administration advisers had been planning to consider a two-dose regimen of Pfizer Inc.’s shot on Tuesday. The delay is to allow the company and its partner BioNTech SE to gather and evaluate more information on the impact of a third dose, agency officials said Friday in a statement.
State Sen. Richard Pan (D-Sacramento) announces a bill to add COVID-19 vaccines to California’s list of required inoculations for attending K-12 schools.
The news is yet another blow to parents who were eager to get their younger kids vaccinated, fueled by concerned around the emergence of new, highly transmissible variants such as Omicron. Enthusiasm for the Pfizer formulation was tempered in December when a study showed two doses failed to meet a laboratory standard for effective immune response in 2- to 4-year-olds, although it did in those from six to 24 months of age.
Data on a third dose are expected in early April, the companies said. That stands to delay U.S. plans for a special vaccination program for kids under age 5. The government has secured sufficient supplies for all 18 million kids from six months through 4 years old, White House coronavirus response coordinator Jeff Zients said Wednesday in a press conference.
In a press call shortly after the announcement, Peter Marks, head of the FDA’s vaccine division hinted that the data at this point was not good enough to merit authorizing the vaccine for young kids, citing the agency’s standards.
“We feel if something does not meet that standard, we cannot proceed forward,” he said on the call, adding that he hoped that the delay “reassures people that the process has a standard.”
He didn’t give more details about what the FDA saw in Pfizer’s two-dose data that caused postponement of a decision until more data come in.
Omicron’s easy spread has many wondering whether they will need COVID-19 boosters every few months or even a new kind of shot altogether.
Pfizer shares fell 0.7% at 2:49 p.m. in New York.
There are already some signs that some parents could be slow to get shots for younger children. COVID-19 vaccinations among kids ages 5 to 11 years have fallen to their lowest levels since the shots were first cleared, according to recent data from the CDC.