Editorial: Widespread coronavirus testing wonât help end the pandemic if itâs inaccurate
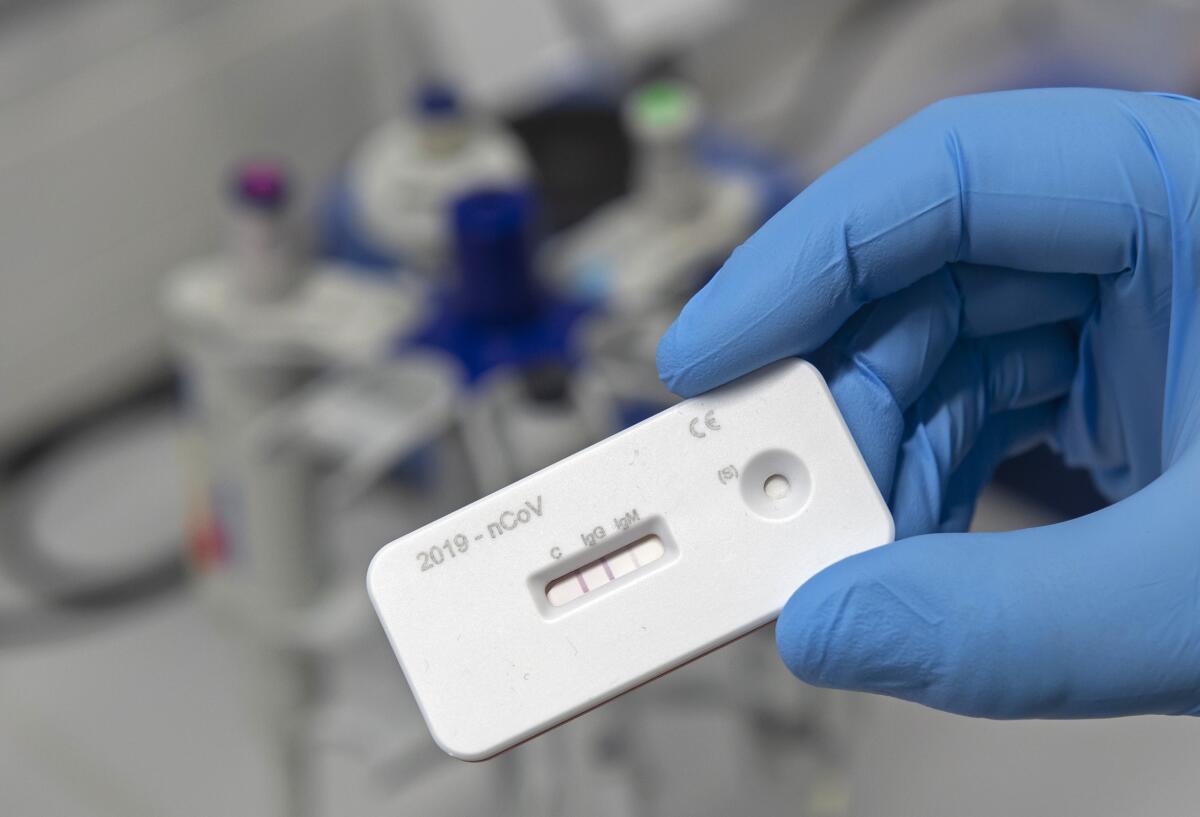
A surge of new coronavirus antibody tests, some of them used in studies that appear to show a far greater rate of infection and recovery than weâd imagined, has set the world to dreaming.
Maybe so many people have been infected that the actual fatality rate from COVID-19 is too low to justify closing down so much of our day-to-day lives. Or maybe a positive antibody test could be used as a kind of âpassportâ to work and normal life, since that person theoretically couldnât be sickened and couldnât pass the virus on to others. Or better yet, maybe we are nearing herd immunity, in which high numbers of immune people keep the disease from sickening the population at large, enabling the public to be set free from their homes.
For now, sorry to say, those are just dreams.
Unlike the diagnostic âPCRâ tests that measure whether someone has contracted COVID-19, antibody tests look at a bodyâs immune system for evidence that an individual has been exposed to and defeated the disease. The nation is awash in more than 120 antibody tests for the novel coronavirus that were approved by the Food and Drug Administration without requiring the usual verification of accuracy. The agency was attempting to get the tests out quickly and avoid a repeat of the unconscionable delays in producing the PCR tests, which remain in too short supply.
But having plenty of tests wonât help if theyâre inaccurate, and some of the antibody tests have unacceptably high rates of false positives. Such mistakes give people the dangerous belief that theyâre safe from the disease and an even more dangerous belief that they canât infect others. Those who show up at a medical office for antibody testing are unlikely to know exactly which test theyâre being given and what its accuracy rate is.
Even with very accurate tests, the presence of antibodies doesnât necessarily mean immunity; too little is known about the resistance that antibodies provide to reinfection. If that immunity exists, itâs also unclear how long it will last.
The studies that purportedly showed that vastly more people have been infected â such as one conducted by Stanford University that concluded the number of infected Santa Clara County residents was 50 to 85 times what had been reported so far â have come under criticisms for alleged shortcomings in their methodology. Itâs not easy to find a random sample of people for antibody testing; Stanford recruited via social media, and the people who volunteered might be skewed toward those who had suffered COVID-19-like symptoms or who knew they had been exposed. The results also were released before the study had been peer-reviewed, which is happening a lot these days because of the pressure to learn more about this new virus.
But even if that many people had been infected, and even if they had immunity as a result, and even if that immunity could be counted on to last at least several months â weâre piling up the âifsâ here â weâd still be woefully short of whatâs needed for herd immunity. Saying â50 to 85 times moreâ sounds like most of the population. In fact, that would add up to, at most, a little more than 4% of Santa Clara County.
And even if COVID-19 has a significantly lower fatality rate than originally thought, that doesnât mean it couldnât kill a lot of people. Given that the virus is easily spread through casual contact and there are no known treatments, if there is no broad-based social distancing, a fatality rate of less than 1% could still exact a horrendous toll.
Antibody tests could still play an important role in understanding the spread of COVID-19. But as with so many aspects of the disease, more attention to science and verification are needed. The FDA needs to figure out which tests are worthy of approval and which arenât, and then allow only the former on the market. More robust studies using those tests are needed to give us a better idea how widespread the infections have been, and those are coming. Indiana is planning one that will involve 20,000 people, with special attention paid to obtaining a more random selection of participants.
And unfortunately, weâll have to wait to find out more about the role of antibodies in preventing future infection, and, we hope, to obtain an effective vaccine from the more than 80 under development. Thatâs our best chance at herd immunity. Yes, science and government need to move quickly against a fast-moving virus, but speed isnât a substitute for reliable information.
More to Read
A cure for the common opinion
Get thought-provoking perspectives with our weekly newsletter.
You may occasionally receive promotional content from the Los Angeles Times.