With gold and rat heart cells, scientists make a robot stingray
- Share via
Here’s a critter that would be a showstopper in your aquarium: By layering rat heart cells over a gold skeleton, scientists have built tiny swimming artificial stingrays that can be driven and guided by light.
These little ray-bots, described in the journal Science, may offer insight into building soft robotics, studying the human heart — and perhaps even building an artificial one from scratch.
Senior author Kit Parker, a Harvard bioengineer, first got the idea for these tiny ray-bots when his young daughter tried to pet a stingray at an aquarium and it quickly and gracefully evaded her hand. Parker watched the rippling body, which reminded him of the stringy cord-like trabeculated muscle on the endocardial surface of the heart, and a thought struck him: He could probably build something that moved like that.
“It kinda hit me like a thunderbolt,” he said. “All the dots connected.”
Parker wasn’t doing this just for kicks: He wanted to understand how the heart works, which is essentially just a muscular pump to push fluid around the inside of a body. And animals such as the stingray or the skate, which have soft bodies that move by generating waves of motion down their bodies, are doing a very similar thing — except they’re acting on fluids outside of the body.
“With the exception of crustaceans, all these marine life forms are muscular pumps. Most of their muscle exists to do one thing: move fluid,” he explained. Parker realized that this animal could make a surprisingly useful physical model. “Do we mimic that in a robotic system and learn more about the mammalian heart, or the human heart?”
It’s an idea that has been gaining traction over the years — after all, there are scientists such as MacArthur “genius” grant recipient John Dabiri at Stanford University, who studies the jellyfish in part because of what its pulsating motion may teach us about the human heart. In fact, Parker had teamed up with Dabiri (then at Caltech) to build a synthetic jellyfish out of rat heart cells, which easily propelled itself around a tank.
See the most-read stories in Science this hour »
But that synthetic jellyfish couldn’t move with any real direction. For this study, Parker and his colleagues took rat heart cells (used because they’re much stronger than, say, human cardiac stem cells) and genetically engineered them to respond to light. They sandwiched that layer together with silicone layers and a tiny skeleton made of gold, partly because gold is relatively easy to sputter onto the silicone rubber and partly because it’s chemically inert and less likely to damage the living cells.
Those cells, layered on the bottom of the pseudo-critter, would pull down. But to mimic the stingray’s undulating forward motion, they needed the motion to pull up as well — which theoretically would require a second layer of cells on top. The problem is that properly synchronizing those two layers’ contractions adds a whole extra layer of complexity.
Instead, the scientists relied on the flexibility of the gold-and-silicone structure, which recoils upward after it’s pulled downward. This allowed for a simple, mechanical solution to an otherwise tricky logistical problem — although designing a material with exactly the right recoil to counteract the heart cells and generate that forward thrust “wasn’t easy,” Parker said.
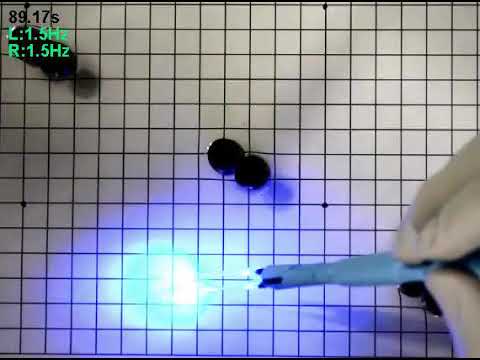
The artificial stringray navigates an obstacle course, guided by a blue light. (Sung-Jin Park)
The result? A tiny soft robot around the size of a penny; it was about a tenth of the size of a little skate, which can fit in the palm of your hand. Weighing in at about 10.18 milligrams, the synthetic ray holds about 200,000 live heart cells in a flexible, 16.3-millimeter body.
When the scientists put the critter in a salt solution (filled with glucose for food) and shone a blue light on it, the heart cells would contract and the ray-bot would swim forward. The robo-ray could also turn left or right, depending on where you shone the blue light (a higher frequency on the right side to turn left, and on the left side to turn right).
Parker says he and his colleagues are already taking lessons from this experiment and applying them to other studies about the heart. He thinks the insights here could be used to make optical pacemakers, instead of electricity-based ones, and he’s excited about what the team has learned about muscle-pumping mechanics. He may even apply what they’ve learned about the robot’s material properties to the “organs on chips” he’s developing in his lab.
But mainly, he said, “I’m excited it worked.”
For now, Parker said he won’t be filling his proverbial aquarium with other rat-celled “species.”
“We’re done,” he said. “This project has served its purpose. We learned what we came to learn.”
But he also sees where these insights might be useful for roboticists, who have long tried to build robots with the efficiency, grace and robustness of living animals.
Traditional robots are typically made of stiff materials such as metal or hard plastic, while living bodies (including our own) masterfully combine both hard and soft elements to make tough but responsive bodies that can navigate unpredictable environments.
Scientists and engineers have made robots inspired by all kinds of animals, including cheetahs and snakes, cockroaches and jellyfish. But making soft robots that can be directed with precision has proved to be a challenge. This new research could offer some helpful pointers on that score, Parker added.
“When you do a project like this and it kind of inspires people, it’s fun,” he said. “This is a transdisciplinary project. And in that way it’s kind of like a piece of art — in that depending on what lens you view this work in, you might see something differently.”
Follow @aminawrite on Twitter for more science news and “like” Los Angeles Times Science & Health on Facebook.
MORE IN SCIENCE
Why scientists are surprised by this strange giant planet and its three suns
Juno arrives at Jupiter: ‘It’s the end of the voyage, but it’s the beginning of the science’
Researchers develop genetic test that can predict your risk of Alzheimer’s disease




