Race for vaccine intensifies as coronavirus hits Asia with a second wave of outbreaks
- Share via
Researchers racing to develop a vaccine for COVID-19 face an even more urgent task in light of recent reports that the coronavirus has rebounded in Asia despite efforts to tamp it down.
New cases of the disease have emerged in Wuhan, China, Singapore and Hong Kong in the past week, after governments lifted some of their social distancing controls.
That raises the stakes for scientists participating in an unparalleled global effort to develop a vaccine, including hundreds in the United States. More than 125 organizations — including major drug companies, government laboratories and top universities — are working on a vaccine or other treatments, according to leading researchers.
“No response has ever gone this fast before,” said Phyllis Arthur, vice president for infectious diseases and diagnostic policy at BIO, a trade association that represents drugmakers, biotech firms and others in the health industry. “We have gone from genetic sequencing to treatment possibilities within weeks.”
These are some of the unusual new scenes across the Southland during the coronavirus outbreak.
Even with a vaccine, COVID-19 will remain a menace, because so much is unknown about it. It is unclear whether the coronavirus lodges itself permanently in recovered persons as do other viruses that remain infectious for decades. And researchers are unsure if the virus could mutate in the years ahead, reducing the effectiveness of a vaccine and forcing it to be modified.
“Don’t expect this to go away soon,” said David Rakestraw, a chemical physicist who is helping lead the COVID-19 work underway at Lawrence Livermore National Laboratory. “The virus is in the population now, and it will evolve.”
In laboratories across the nation, researchers have mobilized like never before to tap new tools in the race for medicines that could treat and prevent the disease.
The 125 organizations run the gamut from the world’s largest pharmaceutical firms to obscure biotech start-ups, said Amy Finan, chief executive of the nonprofit Sabin Vaccine Institute.
“Researchers are working across country lines,” Finan said. “People are working morning, noon and night to come up with solutions.”
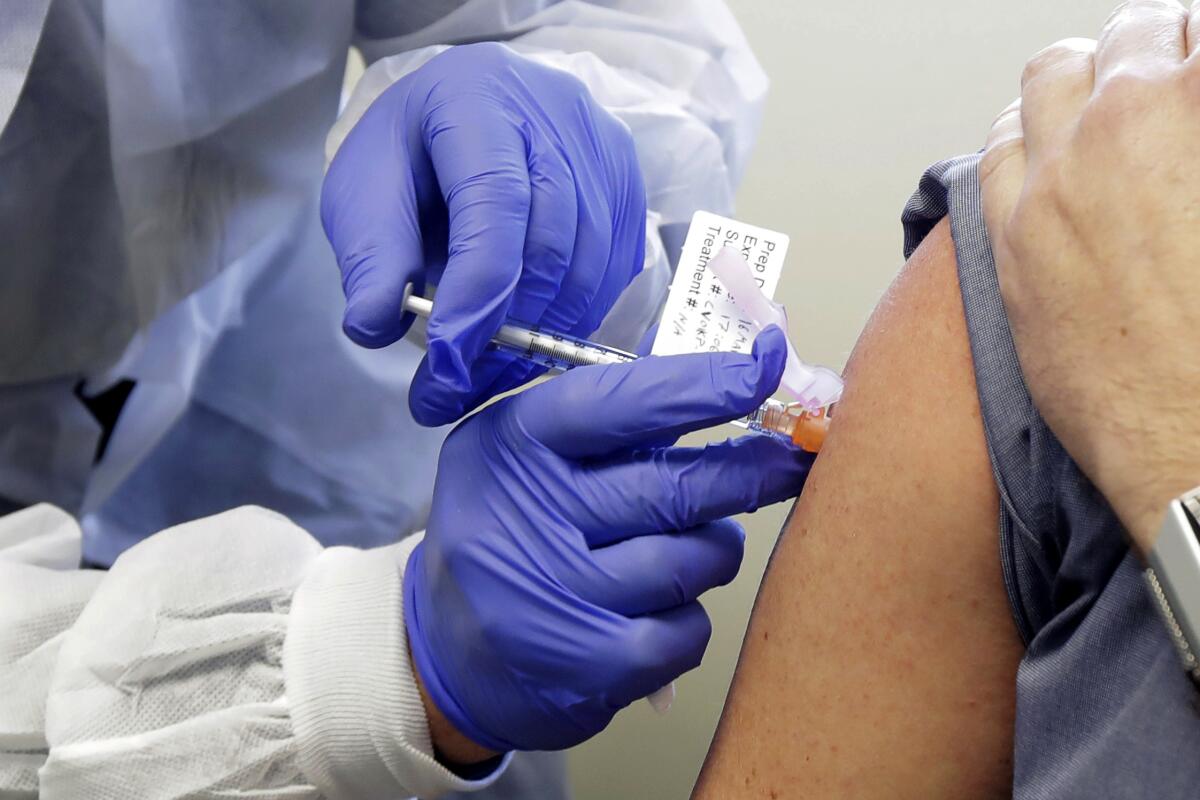
One early stage vaccine trial to determine the drug’s safety began last month in Washington state. Others are forecast to start by early summer. But second stage trials, clearing a drug through regulatory hurdles and setting up a manufacturing base, will take much more time.
How a vaccine or a number of vaccines will be used is not yet clear, Arthur said. It could follow the practice of an Ebola vaccine, which is held in reserve in case of an outbreak but not administered widely. Or it could be like the measles vaccine, which is given to everybody.
The surge in research is largely organic, not commanded or organized by the federal government. Private investment is financing most of the current work. But Congress approved $11 billion in funding for COVID-19 medical response, with $9 billion administered by the government’s Biomedical Advanced Research and Development Authority, Finan said. The money includes a $3.5-billion allocation for new manufacturing facilities, leaving about $5 billion to $6 billion for medical research.
The latest updates from our reporters in California and around the world
The White House’s Office of Science and Technology Policy said in a statement that it is seeking a “whole of America response” that requires the current approach of tapping federal agencies, private industry, academia and the nonprofit sector.
On the federal level, the U.S. national laboratory system has roughly 1,000 scientists and doctors at its disposal, and it operates the world’s largest network of supercomputers.
In the Bay Area, Lawrence Livermore is using computer modeling technologies — developed to guarantee the reliability of nuclear bombs — to examine how 26 million different known molecules might bind and destroy the function of the coronavirus.
To do so, scientists built a virtual 3D model of a virus and started examining how a molecule could bind onto the virus, a modeling job that requires computational power that has come online only in recent years, said Rakestraw, the Livermore scientist. Just one measure of that challenge is the amount of electrical power needed: the lab recently spent $150 million for additional high voltage electrical lines to operate its banks of supercomputers.
Los Alamos National Lab, which developed the first atomic bomb, has been working in biological sciences since 1945 and is applying its earlier pioneering research on HIV and influenza to the new coronavirus, said Kirsten Taylor-McCabe, a biochemist and program manager at the New Mexico lab.
“We do see resurgence and second waves in the future,” she said. “As you ease off restrictions in place and transportation increases, you can see resurgence over time. A vaccine will be very important in preventing a resurgence.”
Among the big actors in the private sector, Johnson & Johnson announced that it has identified a lead vaccine candidate and pledged $1 billion in funding in collaboration with the Biomedical Advanced Research and Development Authority to test and possibly manufacture the drug.
The New Jersey healthcare giant said it expects to initiate “human clinical studies of its lead vaccine candidate at the latest by September 2020 and anticipates the first batches of a COVID-19 vaccine could be available for emergency use authorization in early 2021.”
A number of research efforts are focusing on a strategy of harnessing human antibodies, which are nonliving proteins in the body’s immune system, for both treatments and vaccines.
Amgen, which bills itself as the world’s largest independent biotech firm, announced last week that it would jump into the challenge in partnership with Adaptive Biotechnologies to develop such antibodies that could give several weeks of protection to the healthy, said Dr. David Reese, executive vice president of research and development.
The company, which has 8,200 employees at its Thousand Oaks headquarters, is using high-speed computers to screen millions of human cells from recovered virus patients to identify possible antibodies effective against the virus, he said.
“We view this as the greatest public health challenge in a century that the world has faced,” Reese said.
Reese and others say the amount of funding is adequate to move as quickly as possible, though a manufacturing capability to turn out billions of doses will require a much larger industrial enterprise.
Meanwhile, a team in China gained attention this week when it announced a plan to begin phase 2 clinical trials, which would test the effectiveness of a vaccine on human subjects. CanSino Bio and Academy of Military Medical Sciences’ Institute of Biotechnology said the study would examine a DNA-type vaccine “soon” in a filing to the Hong Kong Stock Exchange.
Inovio, a U.S. biotech firm with research labs in San Diego, has launched phase 1 studies of a vaccine. It will administer a sequence of DNA molecules to the human body that will try to attack the virus by attaching antibodies on its spike structures, said the firm’s research chief Dr. Kate Broderick.
Broderick said there remains significant gaps in scientific knowledge of the virus, such as exactly why some exposed individuals do not get the disease or whether the virus goes dormant inside people who don’t suffer symptoms. With some viral diseases, such as herpes, the virus remains present throughout life.
But she is “completely confident” that at some point a vaccine will be available and that likely a suite of vaccines will aid in the battle against the disease.
But there’s little chance of a near-term miracle, Broderick and other experts say. A vaccine remains 12 to 18 months into the future, but treatments for the infected may be coming along much faster, possibly within this year.
Creating a vaccine capable of preventing the coronavirus that causes COVID-19 will take many months — if not a year or more. These are the steps involved.
Once a vaccine is successfully developed and tested, the government is likely to move cautiously in approving it for general use. In a crisis like the current one, the Food and Drug Administration has the ability to issue an “emergency use authorization,” though it might be limited to people on the front lines of the battle against the disease.
By historical standards, 12 to 18 months would be light speed. The first U.S. polio epidemic occurred in 1894, but it continued to maim thousands yearly until Albert Sabin licensed the current safe oral vaccine in 1960. Sabin’s discovery resulted in the elimination of the crippling disease in the U.S. in 1994.
No one in the research establishment expects that the world will be social distancing for decades.
“We are committed to moving as fast as humanly possible,” said Reese, the Amgen research chief.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.