Botox maker Allergan sued by Las Vegas surgeon
A Las Vegas plastic surgeon has sued Allergan Inc., accusing the Irvine pharmaceutical company of selling its popular wrinkle treatment Botox in large vials and encouraging physicians to unsafely reuse them.
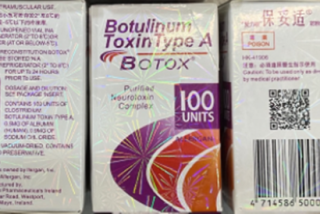
Dr. Julio L. Garcia contends that Allergan sold Botox exclusively in 100-unit vials for several years, even though a typical treatment required just 20 units. The lawsuit, filed in federal court in Los Angeles, said Allergan sales representatives encouraged physicians to use the vials on multiple patients, a practice now condemned by health officials.
The lawsuit accuses Botox of false advertising and violating Californiaâs unfair competition law. It seeks unspecified monetary damages and certification as a class action. The suit does not contend that any Botox patients became ill because of the alleged safety violations.
Allergan spokeswoman Caroline Van Hove declined Monday to discuss specific allegations in the lawsuit. She said the company advocates the single use of Botox vials, as its labels indicate.
Botox is the brand name for Botulinum Toxin Type A, a toxin that temporarily weakens facial muscles that contribute to wrinkles. Botox Cosmetic was available only in 100-unit vials from 2002 until 2008, when Allergan started selling Botox in 50-unit vials.
To defer patient costs, Allergan sales representatives told doctors to promote a âbuddy system,â in which two or more patients came into the office together to share a single vial, Garcia said in the lawsuit.
The Centers for Disease Control and state public health agencies have warned against using medication from a single vial to treat multiple patients.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production â and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.