Patients sue Gilead, saying drug company intentionally delayed safer HIV medicine
- Share via
Two Southern California men filed suit against Gilead Sciences on Tuesday, saying they were harmed when the drug company intentionally delayed development of a safer version of a crucial HIV medicine so that it could continue to profit from its lucrative monopoly.
The lawsuit — and a similar case that seeks class-action status — says that Gilead executives knew as early as 2000 that the company’s scientists had developed a less toxic form of its HIV medicine tenofovir that was less harmful to patients’ kidneys and bones.
But instead of continuing to develop the safer alternative, the lawsuit claims, the Foster City company decided to hide tenofovir’s risks while earning billions of dollars as it became one of the world’s most prescribed medicines for HIV.
A Gilead spokesman said Tuesday the company was thoroughly reviewing the complaints and would not comment until that process is complete.
The lawsuit says that HIV patients suffered from as many as 10 years of “additional accumulated kidney and bone toxicity” while using the drug as the company kept the safer version on a shelf in its lab.
“A company I trusted with my life took advantage of that trust by misrepresenting the side effects,” said Michael Lujano, a Los Angeles County resident who is one of the plaintiffs. “Gilead shelved a far safer drug … simply to increase its long-term profits.”
The lawyers for the men bringing the personal injury lawsuit are being funded by the AIDS Healthcare Foundation, a nonprofit headquartered in Los Angeles. They filed a parallel case Tuesday that seeks class-action status for all California patients who took the medicine from Oct. 26, 2001, until the present.
Earlier the foundation filed a lawsuit attempting to invalidate the drug’s patents, which is on appeal in federal court.
The drug’s history goes back to the 1980s, when it was discovered by European scientists. Gilead, then a small biotech firm, bought the rights to sell the drug. In 1997, the company showed that it fought HIV.
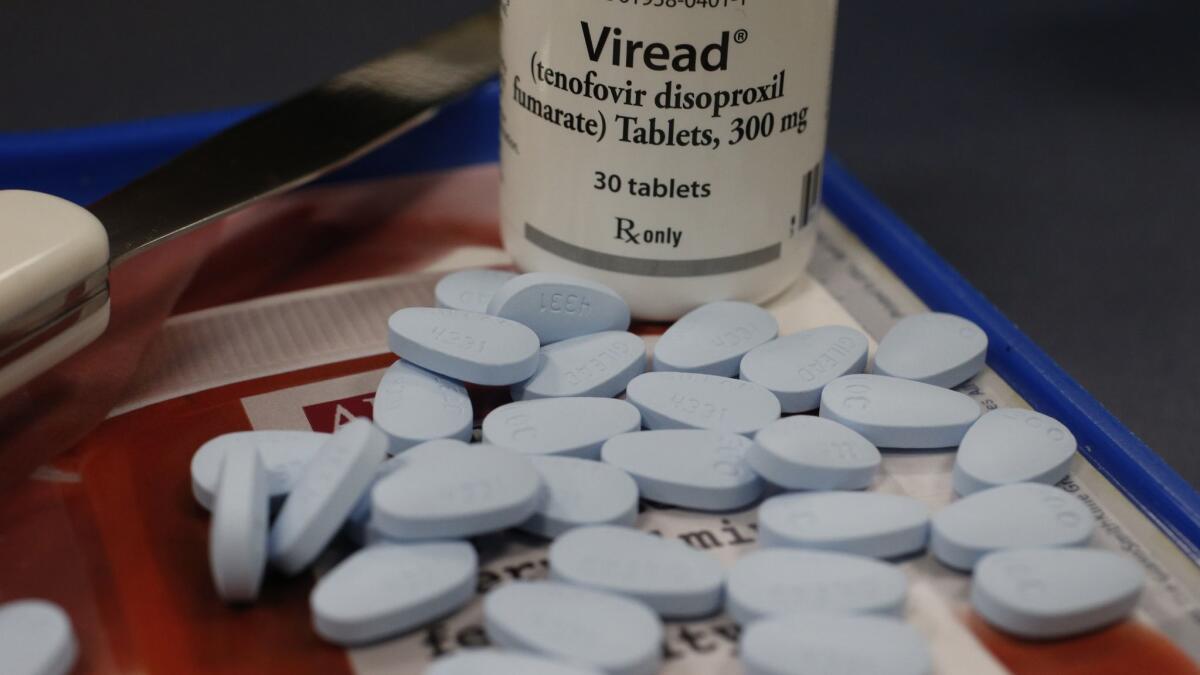
The original formulation of the drug held little sales potential, however, because it had to be given intravenously. Gilead scientists modified its chemical composition to create a drug that could be taken orally. That medicine — called tenofovir disoproxil fumarate, or simply TDF — was approved by the federal Food and Drug Administration in October 2001.
It was originally sold under the brand name Viread. Later it was combined with other HIV medicines and sold under additional brand names, including Atripla, Truvada, Stribild and Complera.
The lawsuits say that Gilead knew when Viread was approved in 2001 that it had to be given in high doses to be effective, which meant it could damage the kidneys and bones. Yet the company failed to adequately disclose those dangers in the medicine’s label, the lawsuits say.
At the time of the drug’s approval, Gilead scientists were already working to reduce its adverse effects. In April 2001, the scientists published research on a different chemical version of the medicine called tenofovir alafenamide fumarate, or TAF.
That animal study showed TAF had a thousand-fold greater activity against HIV than the original medicine invented in Europe, raising the possibility that it would have far less toxicity.
Gilead then paid doctors across the country to give TAF to patients in small clinical trials. The positive results of those studies were not published for years — secrecy that the lawsuits filed Tuesday say was “an act of extreme malice.”
Instead, in October 2004, Gilead abruptly announced that it was ending research on TAF after an “internal business review.” And it continued to pour money into selling the older drug, which was bringing in billions of dollars each year.
The company also continued to quietly apply for new patents on TAF — the drug it had said it would no longer be developing.
More than six years after that 2004 announcement, with the older drug’s patent running out, an executive told investors about “an interesting new molecule” the company had added to its research plans. That drug was TAF. The company then began publishing the results of the earlier studies.
In November 2015, the FDA approved TAF in a combination pill with three other medicines. The drug was called Genvoya.
Other new pills also containing TAF include Odefsey and Descovy.
The company’s sales force is now urging doctors to switch their patients to the new drugs to reduce possible harm to their kidneys and bones.
The lawsuits claim that Gilead delayed the development of TAF in order to extend the number of years where patents shielded the medicines from competition, allowing it to charge high prices.
“By holding on to its research and shelving TAF, Gilead could patent TAF separately and save it for development when their patent and exclusivity on TDF ran out, in 20 years,” the lawsuit claims.
Lujano, one of the plaintiffs, said he took medicines containing the older TDF from 2004 to 2015. In 2016, at age 35, Lujano was diagnosed with osteopenia and osteoporosis of the spine, neck and hip.
The other plaintiff in the personal injury suit is Jonathan C. Gary of San Diego County, who took the drug for 10 years, beginning in 2001. Gary was diagnosed with Fanconi syndrome, a rare kidney disorder, in 2010. Last year, he was diagnosed with osteopenia and osteoporosis at age 59.
The lawsuits, both filed in Los Angeles Superior Court, seek damages for those harmed by the drugs.
The AIDS Healthcare Foundation, which owns clinics that care for HIV patients, says it isn’t seeking to recover money for itself other than reimbursement of attorneys’ fees.
In 2016, the foundation filed a similar lawsuit against Gilead, arguing that the company had intentionally delayed research on the safer form of the medicine in order to keep out competition and preserve its high prices. That case asked the court to invalidate the patents protecting the new drug TAF.
A judge ruled in July 2016 that Gilead had not illegally manipulated the U.S. patent system. The foundation then appealed. That lawsuit is pending in the U.S. Circuit Court of Appeals in Washington.
Follow @melodypetersen on Twitter
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.











