FDA allows gay and bisexual men to donate blood, but only under certain conditions
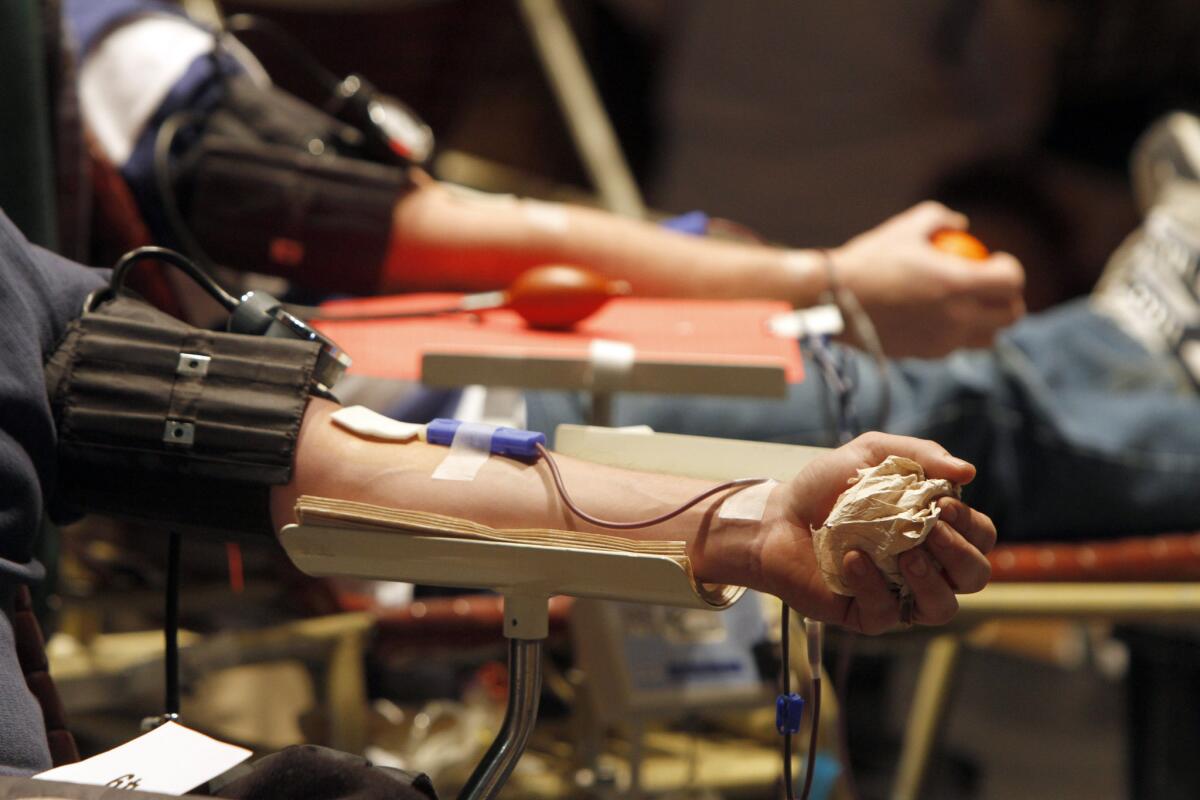
Donors give blood at a blood drive. Gay and bisexual men will be allowed to join them under a new FDA policy announced Monday, but only if they are HIV-free and have abstained from sex with other men for at least a year.
The U.S. Food and Drug Administration has officially ended its policy that blocked gay and bisexual men from donating blood.
The new policy, announced Monday, allows these men to donate blood on the condition that they are HIV-free and have not had sex with another man for at least one year.
The one-year deferral treats men who have sex with men the same as people in other groups that face an increased risk of having the human immunodeficiency virus, which causes AIDS. For instance, people who received a blood transfusion must wait one year before donating blood. The same goes for those, such as medical workers, who accidentally came into contact with someone elseâs blood through an open wound or a needle stick.
Men and women who have ever tested positive for HIV continue to face an indefinite ban on becoming blood donors, as do people with a history of non-prescription injection drug use and people who have ever exchanged sex for money or drugs, according to the new FDA policy.
âThe FDAâs responsibility is to maintain a high level of blood product safety for people whose lives depend on it,â said Dr. Stephen Ostroff, the FDAâs acting commissioner, in a statement. âWe have taken great care to ensure this policy revision is backed by sound science and continues to protect our blood supply.â
The federal agency did not make the change lightly. It spent several years evaluating epidemiological data from around the world and using statistical models to determine whether the lifetime ban on blood donations from men who have sex with men could be safely eased.
Late last year, an independent panel of experts concluded that a one-year deferral would not bring added risk to the blood supply. A few weeks later, the FDA said it planned to change its policy to allow gay and bisexual men to donate blood for the first time since the early days of the AIDS epidemic.
The new rules bring the United States in line with Britain, Sweden, Japan and Australia, all of which have dropped lifetime bans on men who have sex with men in favor of one-year deferrals.
After Australia made the switch from an indefinite ban to a one-year deferral in 2000, researchers tested more than 8 million samples of donated blood and found no change in the safety of the countryâs blood supply, according to the FDA. No studies have evaluated whether a shorter deferral period would also be safe, the agency noted.
The FDA added that its efforts to protect the nationâs blood supply â including deferrals for members of high-risk groups and more accurate tests to screen donor blood â have drastically reduced the risk of spreading HIV through blood transfusions. That risk is now 1 in 1.47 million, down from 1 in 2,500 in the mid-1980s, before HIV testing became available.
The FDAâs policy is also aimed at preventing the spread of hepatitis B and C, syphilis and other sexually transmitted viruses through blood transfusions.
The agency said it would continue to evaluate new scientific data as they become available and make further modifications to its blood donation policy as needed.
Follow me on Twitter @LATkarenkaplan and âlikeâ Los Angeles Times Science & Health on Facebook.







