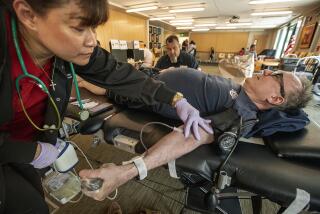
FDA proposes letting gay men donate blood, with some caveats
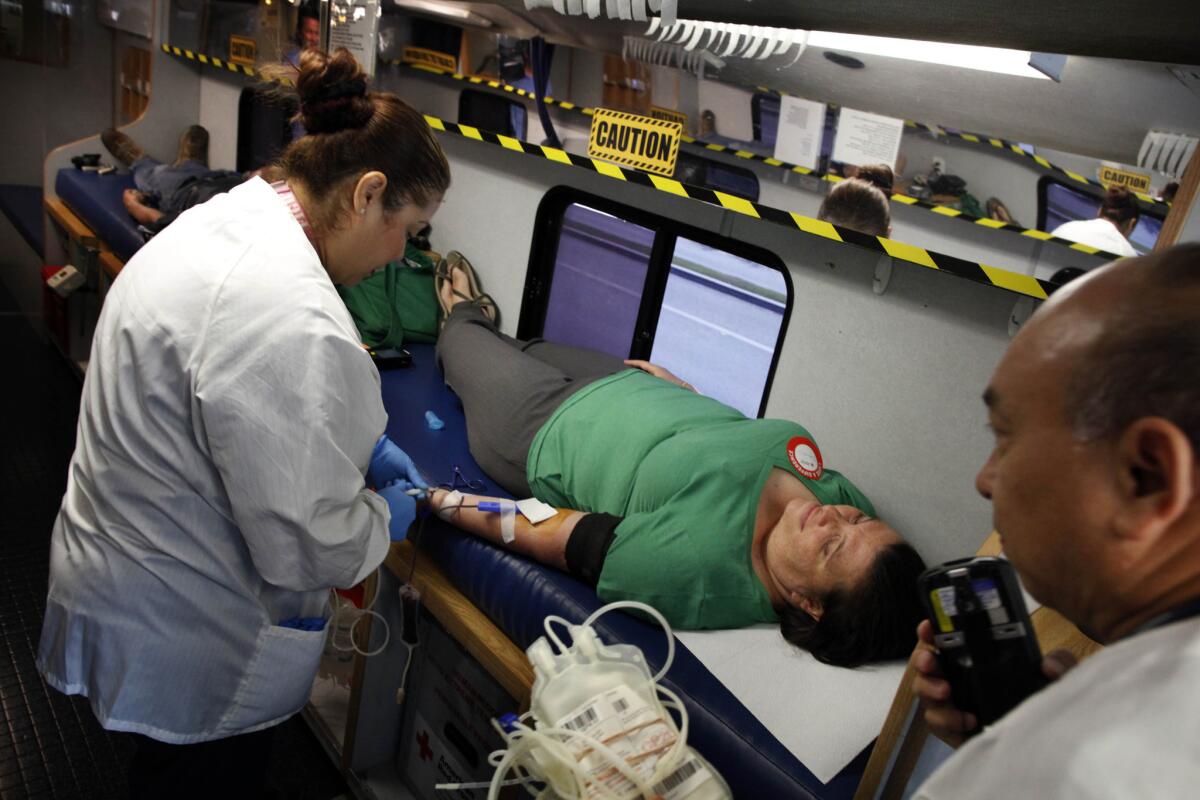
Phlebotomist Nancy Del Campo collects blood from Jessi Baden at a
The Food and Drug Administration proposed new rules Tuesday that would allow gay and bisexual men to donate blood in the U.S. for the first time in decades.
In a move that has been anticipated for months, the FDA released draft regulations that would eliminate the blanket ban on donor blood from any man who has had sex with another man at least once since 1977. Instead, such men would be allowed to donate blood as long as they are healthy and have not had same-sex relations for at least one year.
Several other countries, including Australia, Sweden, Britain and Japan, have already adopted one-year deferral policies for gay and bisexual men.
The ban, instated in 1985 in the early years of the AIDS epidemic, has long been criticized by members of the medical community and portrayed by LGBT groups as a civil rights issue.
The new rules would bring the requirements for gay and bisexual men more in line with those for other groups that engage in what is considered risky sexual behavior.
Currently, people who have had heterosexual sex with an HIV-infected partner, an intravenous drug user or a sex worker are permitted to donate blood only after waiting one year and testing negative for HIV. Travelers who have been to certain parts of Africa are also deferred as blood donors for at least a year after they return to the United States.
But healthy gay men in monogamous relationships â as well as gay men who havenât had sex with men since 1977 â are not allowed to donate blood.
Last year, a panel of independent experts concluded that imposing a yearlong waiting period would not endanger the safety of the nationâs supply of donated blood. The American Medical Assn. also voted last year to oppose the ban, calling it âdiscriminatory and not based on sound science.â
The three groups that supply nearly all of the nationâs blood â the American Red Cross, AABB and Americaâs Blood Centers â have opposed the ban for years.
Although activists have decried the lifetime ban on gay and bisexual male donors, calling it outdated and homophobic, the prevalence of HIV in the gay community continues to be a concern as it relates to blood donations.
According the Centers for Disease Control and Prevention, men who have sex with men make up only 7% of the U.S. male population, yet they made up 57% of people living with HIV in 2011, the most recent year for which data were available.
The FDA, which regulates how blood donations are collected and how blood is transfused, said last year that it would make sure the nationâs blood supply remained safe by implementing a blood surveillance system when it rolls out its new, more relaxed rules.
Activists praised the proposed rules Tuesday but reiterated support for moving toward policies that lean on individual risk assessment more than sexual orientation.
âWe are pleased to see the FDA has issued the draft guidance,â the National Gay Blood Drive said on its website. âWe will continue to encourage the FDA to move toward a deferral based upon individual risk assessment.â
The FDA will take comments on the draft proposal for 60 days before beginning to finalize the guidelines, according to the Associated Press.
For more breaking news, follow me @cmaiduc